Expand more
ABOUT ALLMED

Our History
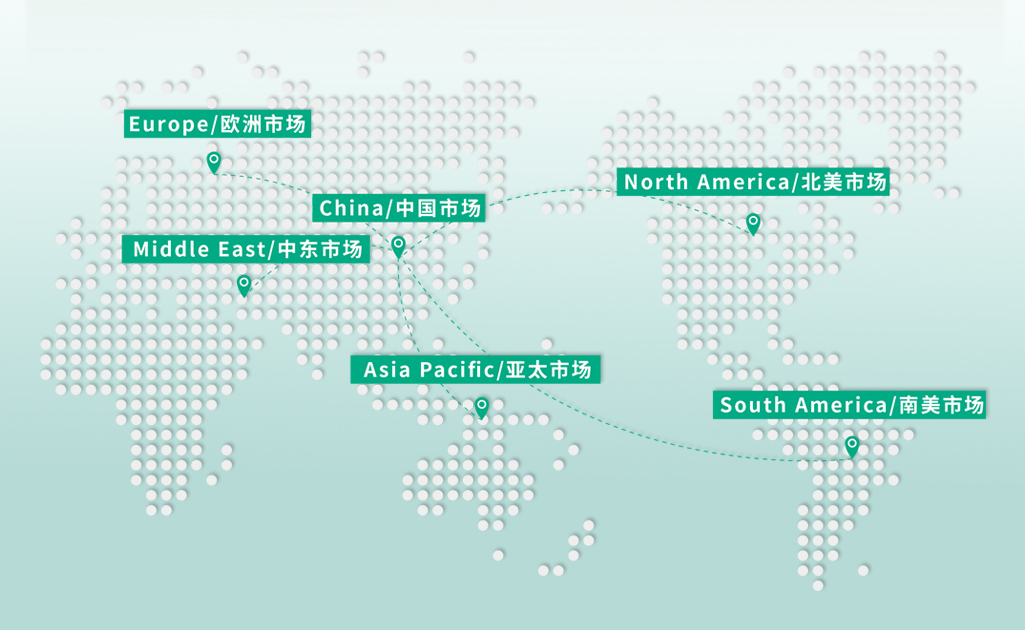
Allmed Medical Products Co., Ltd is a leading supplier in medical dressings and infection control solutions globally, with products delivered to more than 70 countries and regions across six continents.

Qualification And Honor
Allmed Medical holds international certifications including CE, ISO13485, CNAS, CSR, and FSC, demonstrating its commitment to excellence in quality and social responsibility.
PRODUCT SOLUTIONS
NEWSROOM
Events
Stay updated on Allmed Medical's latest exhibition and event initiatives, and witness our innovation and growing influence in the global healthcare industry.

 Learn More +
Learn More +